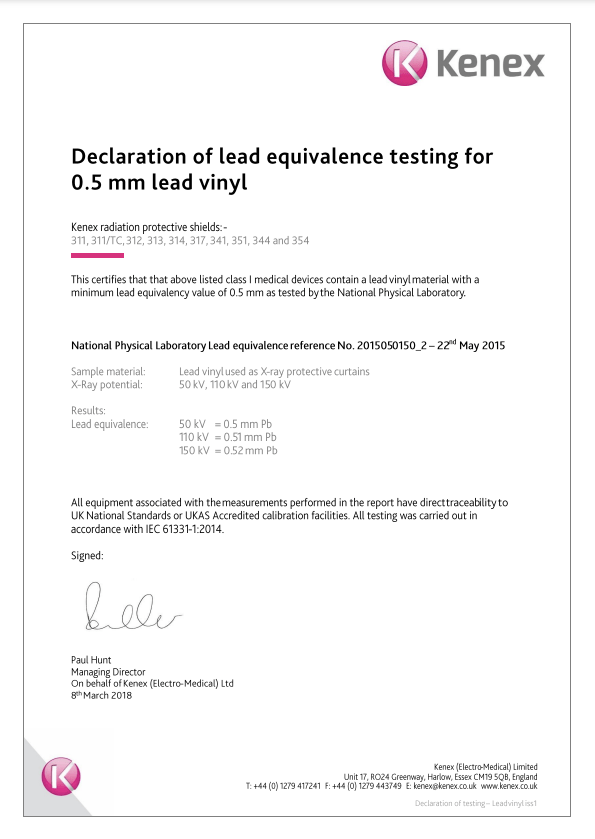
Compliance and certification
Equipment produced by Kenex is noted for its effectiveness and ease of use. These characteristics are the result of a long process, including research & development, ergonomic design, and advanced manufacturing processes. The compliance and certification we have attained demand the very highest standards, including full traceability for every component, enabling us to track the life of each product. In addition, each product is tested and independently checked to ensure it is fit for its purpose.

ISO 13485:2016 (QMS for regulation of medical devices)

EU 2016/425 Module D 0598 (PPE)

UK 2016/425 Module D 0120 (PPE)

EU TYPE-Examination certificate, Module B

UK TYPE-Examination certificate, Module B

Declaration of Conformity EU MDR 2017.745

Declaration of Conformity UK MDR 2002

Declaration of Conformity PPE 2016/425

RoHS & REACH declaration issue 6
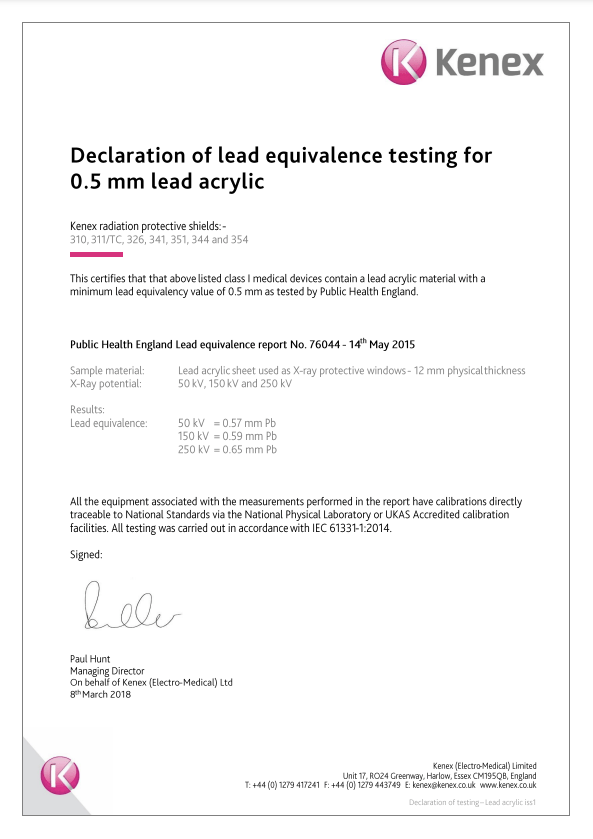
Declaration of testing for lead acrylic

Declaration of testing for lead glass